Research Findings
A groundbreaking study published in the journal "Aging" demonstrated that HBOT could increase telomere length by 20% in certain immune cells. The study, which involved participants receiving daily HBOT sessions over several months, also showed improvements in other aging-related markers.
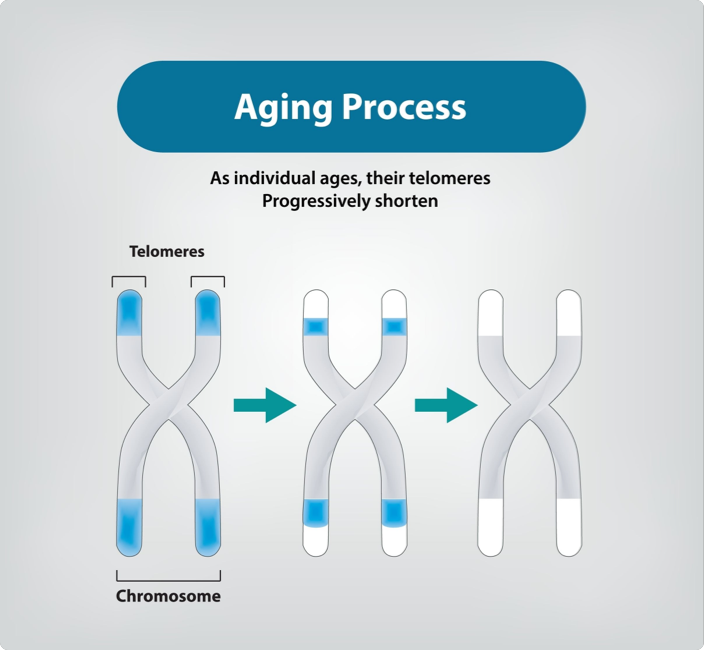
The Connection Between Telomeres and Aging
As we age, our telomeres naturally become shorter with each cell division. When telomeres become too short, cells can no longer divide properly and may either stop functioning (cellular senescence) or die. This progressive shortening of telomeres is considered one of the primary mechanisms of biological aging and has been linked to various age-related diseases and conditions, including:
• Cardiovascular disease
• Certain types of cancer
• Decreased immune function
• Cognitive decline
• Reduced tissue repair capability
Scientists have found that individuals with longer telomeres tend to have better health outcomes and increased longevity, leading researchers to explore ways to maintain or even lengthen telomeres.
Implications for Anti-Aging Medicine
The discovery that HBOT might influence telomere length opens new possibilities in the field of anti-aging medicine. However, it's important to note several key considerations:
1. Treatment Protocol: The benefits observed in research studies typically required consistent, long-term HBOT sessions under medical supervision, such as that we provide at Bay Area Hyperbarics.
2. Individual Variation: Response to HBOT can vary significantly between individuals, and not everyone may experience the same degree of telomere lengthening.
3. Complementary Approach: HBOT should be considered as part of a comprehensive approach to healthy aging, including proper nutrition, regular exercise, and stress management.
Understanding Telomeres and Their Function
At the end of every chromosome in our cells lies a protective structure called a telomere. These repetitive DNA sequences, often compared to the plastic tips on shoelaces, serve a crucial function: they protect our genetic material during cell division. Each time a cell divides, these telomeres become slightly shorter, acting as a biological clock that measures cellular age.
Think of telomeres as a buffer zone that prevents the loss of essential genetic information during replication. Without telomeres, each cell division would result in the loss of critical genetic data, much like trying to photocopy a page where the edges keep getting cut off with each subsequent copy.
The Mechanism of Action
The relationship between HBOT and telomere lengthening appears to work through several pathways:
1. Enhanced DNA Repair: The increased oxygen pressure stimulates cellular repair mechanisms, including those involved in maintaining telomere integrity.
2. Reduced Oxidative Stress: While it might seem counterintuitive, controlled exposure to high oxygen pressures can actually trigger antioxidant responses in the body, helping to protect telomeres from damage.
3. Stem Cell Activation: HBOT has been shown to mobilize stem cells, which typically have higher telomerase activity – the enzyme responsible for maintaining telomere length.
Looking to the Future
While the connection between HBOT and telomere lengthening represents an exciting development in anti-aging research, more studies are needed to fully understand the long-term implications and optimal treatment protocols. The potential to influence biological aging through telomere modification could lead to new therapeutic approaches for age-related diseases and perhaps even extend healthy human lifespan.
As research continues, HBOT may emerge as a valuable tool in the growing arsenal of anti-aging interventions. However, it's essential to approach these developments with measured optimism and continue to support research that can help us better understand the complex relationship between telomeres, aging, and therapeutic interventions.